
Differences in atomic shape and size determined the various properties of matter. The atomic philosophy of the early Greeksĭemocritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite numbers through empty space until stopped. Electrons move around a nucleus, but only in prescribed orbits, and if electrons jump to a lower-energy orbit, the difference is sent out as radiation.

In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values. For decades, the proton was considered an elementary particle. It is 100 years since Ernest Rutherford published his results proving the existence of the proton. Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom. So, he not only discovered the electron but determined it was a fundamental part of an atom. In 1904, Thomson proposed a model of the atom as a sphere of positive matter with electrons positioned based on electrostatic forces. In Thomson’s model, the atom is composed of electrons (which Thomson still called “corpuscles,” though G. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In addition, he also studied positively charged particles in neon gas. He demonstrated that cathode rays were negatively charged. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube.
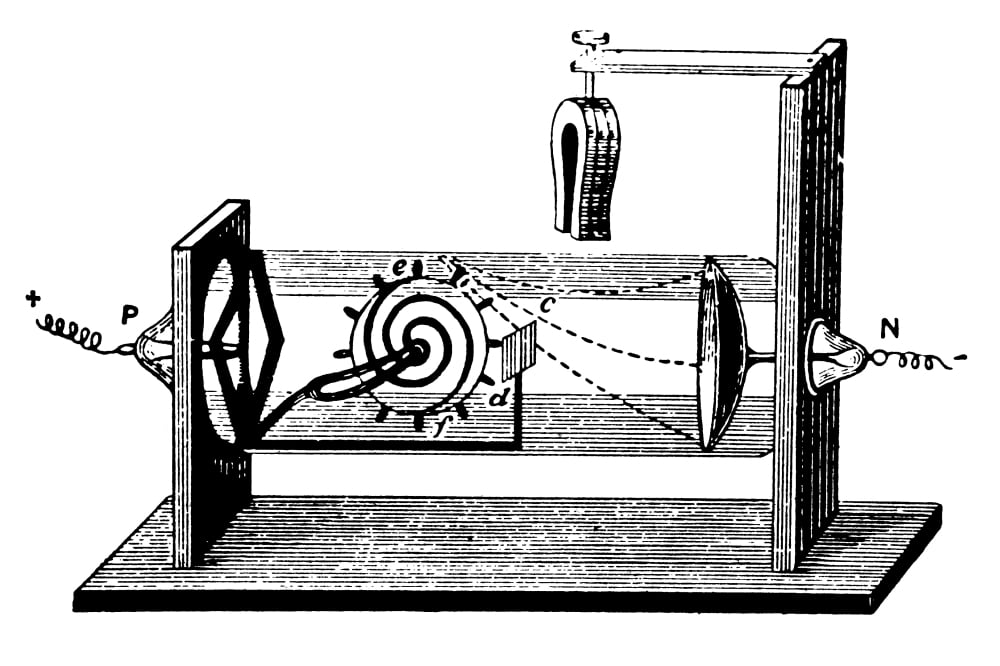

Every Hydrogen Atom has only one Electron.Discovery of the Electron – The first subatomic particle.


 0 kommentar(er)
0 kommentar(er)
